Detailed Program(2021)
HOME > Conference > Detailed Program(2021)GBC1
Opening Ceremony / Keynote & Plenary Session
Forum Overview
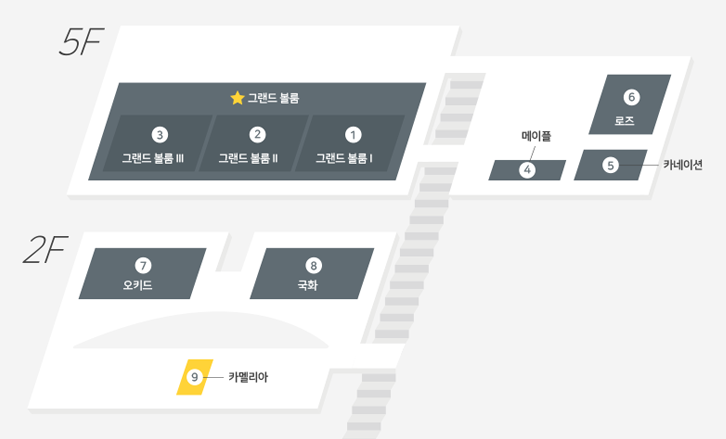
| Date/Time | 9.13(Mon) 13:30 - 18:00 | Location | Grand Ballroom I,II,III (5F) |
|---|---|---|---|
| Overview |
A New Normal: A Leap towards Newly Found Future of Biopharmaceuticals With a global paradigm shift in the health-care industry, where people turn more toward preventive measures than treatments, living a “healthy” long life is far more important than living “longer.” With this flow, biopharmaceuticals take center stage with their key potential in combining biology and advanced technologies to cope with a novel infectious disease and rare incurable disease. Now that we are on this shifting tide, it is imperative to take measures for biopharmaceuticals’ leap toward the newly found future. At the GBC 2021, world leaders in biopharmaceuticals gather to discuss “What is the current situation that biopharmaceuticals are facing NOW?” “What are we expecting NEXT?” and “What is the future and BEYOND?. In addition, there will be further discussions on the sustainable future and insights on advanced biopharmaceuticals toward the “New Normal” and even the “Next Normal.” |
||
Program
| Time | Program | Speakers | Files | |
|---|---|---|---|---|
| Keynote Speech : A New Normal: A Leap towards Newly Found Future of Biopharmaceuticals | ||||
| 14:00~14:15 | 15’ | Peter Doherty (Peter Doherty Institute at the University of Melbourne) |
||
| 14:15~14:55 | 40’ | [Plenary] Preparedness and response against new pandemic in future | Jong-koo Lee (Seoul National University, College of Medicine/Hospital) |
|
| 14:55~15:35 | 40’ | Robert S. Kerbel (Sunnybrook Research Institute) |
||
| 15:35~15:55 | 20’ | Margaret Hamburg (Fomer FDA commisioner /Nuclear Threat Initiative) |
||
| 15:55~16:35 | 40’ | Aaron Ciechanover (Faculty of medicine at the Technion - Israel Institute of Technology) |
||
| 16:35~17:00 | 25’ | Rogério Gaspar (WHO) |
||
| 17:00~17:20 | 20’ | Larry W. Kwak (City of Hope Comprehensive Cancer Center) |
||
| 17:20~18:00 | 40’ | The public private partnerships utilized in the US Government program for COVID vaccine development | Lawrence Corey (Fred Hutchinson Cancer Research Center) |
|
GBC2
Vaccine Forum
Forum Overview
| Date/Time | 9.14(Tue) 09:00 – 12:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
Widespread adoption of novel platform-based vaccines and global vaccine strategies and regulatory directions in Next Normal era Although the whole world is still greatly suffering from COVID-19, multiple vaccines and treatments for COVID-19 have contributed to the concerted efforts to overcome the pandemic. However, expectations and concerns still coexist as to the stable supply of COVID-19 vaccines and treatments, especially so for vaccines using new platforms. In tune with ongoing shifts, Korea is focused on vaccine development to adapt to evolving healthcare trends and prepare for the Next Normal era. With all of this in mind, GBC 2021 Vaccine Forum will share the perspectives of overseas regulatory authorities and information on global vaccine development with respect to securing vaccine platforms that will enable a rapid response to emerging diseases and developing key source technologies for next-generation vaccines, etc. |
||
Program
| Time | Program | Speakers | Files | |
|---|---|---|---|---|
|
Chair: Jin Han Kang
Novel platform-based vaccines and global vaccine strategies and regulatory directions in Next Normal era |
||||
| 09:10~09:50 | 40’ | Jerome Kim (International Vaccine Institute ) |
||
| 09:50~10:00 | 10’ | Break | ||
| 10:40~11:20 | 40’ | Jae-Hwan Nam (The Catholic University of Korea) |
||
| 10:00~10:40 | 40’ | COVID 19 vaccine development and the future of mRNA strategy | Randall Hyer (Moderna) |
|
| 11:20~11:30 | 10’ | Break | ||
| 11:30~12:10 | 40’ | Jinho Shin (WHO) |
||
| 12:10~12:50 | 40’ | Ralf Wagner (PEI) |
||
GBC3
Recombinant Protein Products Forum
Forum Overview
| Date/Time | 9.14(Tue) 14:00 – 18:00 | Location | Grand Ballroom II (5F) |
|---|---|---|---|
| Overview |
Compelling efforts to develop treatments in the COVID-19 era Vigorous efforts to develop treatments against COVID-19 are now being made as the world continuously suffers from the pandemic situation. The Recombinant Protein Products Forum will discuss the status and the future outlook of COVID-19 antibody treatments. Furthermore, by sharing practical examples, we’ll discuss the role that should be played by regulatory authorities in these development strategies. |
||
Program
| Time | Program | Speakers | Files | |
|---|---|---|---|---|
| Efforts for rapid development of biotherapeutics in the COVID-19 era | ||||
| 14:00~14:45 | 45’ | John R Dobbins (Lilly) |
||
| 14:45~15:30 | 45’ | Junho Chung (Seoul National University College of Medicine) |
||
| 15:30~16:15 | 45’ | Minsoo Kim (Celltrion) |
||
| 16:15~16:30 | 15’ | Break | ||
| 16:30~17:15 | 45’ | Alexander Liakos (GSK) |
||
| 17:15~18:00 | 45’ | Marjorie A. Shapiro (FDA) |
||
GBC4
Advanced Biopharmaceuticals Forum
Forum Overview
| Date/Time | 9.15(Wed) 14:00 – 18:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
Developments and regulations in stem cell therapy and xenotransplantation The advanced biopharmaceuticals industry is rapidly growing as it has shown the potential to treat rare and incurable diseases. Stem cell therapy has garnered significant attention as new treatments, considering their potential application to various diseases. Xenotransplantation products have also been regarded as potential alternatives in which donated organs are short in spspsupply. Despite the high interest and expectation, technical limitations, safety issues, and ethical challenges still prevail. At The Advanced Biopharmaceuticals Forum, the latest information including development trends and future outlook on induced pluripotent stem cell therapy, embryonic stem cell therapy and xenotransplantation will be shared. With this information, we’ll look into their possibilities and limitations. |
||
Program
| Time | Program | Speakers | Files | |
|---|---|---|---|---|
| Session1: Present and future of cell therapy with using stem cell | ||||
| 14:00~14:40 | 40’ | Current Trends of Cell Therapy Development Using induced Pluripotent Stem Cells (iPSC) | Jihwan Song (CHA University) |
|
| 14:40~15:20 | 40’ | Shin Kawamata (FBRI, Japan) |
||
| 15:20~16:00 | 40’ | Glyn Stacey (ISCBI) |
||
| 16:00~16:10 | 10’ | Break | ||
| Session2: Trends on transplantation study using xenotransplantation | ||||
| 16:10~16:50 | 40’ | Wayne Hawthorne (Sydney University/IXA) |
||
| 16:50~17:30 | 40’ | Sung Joo Kim (GenNBio) |
||
GBC5
Blood Products Forum
Forum Overview
| Date/Time | 9.15(Wed) 09:00 – 13:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
Regulatory Environment and Development Direction of Blood Products and Plasma Derivatives The blood supply through blood donation is predicted to reduce with global environmental changes, such as the outbreak of a novel infectious disease like COVID-19, population decrease, and climate change. Thus, we will share the current situation of approval and safety management system of blood products in compliance with WHO’s principles of self-sufficient blood supply and banning commercial use of blood products, which cover medications derived from blood. In addition, we will also discuss the recent Korean and international trends of policies and good manufacturing practice (GMP) guidance on blood products, as well as the direction of development. |
||
Program
| Time | Program | Speakers | Files | |
|---|---|---|---|---|
| Regulatory Environment and Development Direction of Blood Products and Plasma Derivatives | ||||
| 09:00~09:40 | 40’ | Dong Hi Hwang (Korean Red Cross Plasma Fractionation Center) |
||
| 09:40~10:20 | 40’ | Seungjoo Kim (SK PLASMA) |
||
| 10:20~10:35 | 15’ | Break | ||
| 10:35~10:55 | 20’ | Sungwon Jung (Baxter Korea) |
||
| 10:55~11:15 | 20’ | Biological Products: Human Blood and Blood Components | Gavriel Yifat (J&J) |
|
| 11:15~11:55 | 40’ | Safety Control Plasma-Derived Products in Korea | Sang Min Shin (Green Cross Corp.) |
|
| 11:55~12:10 | 15’ | Break | ||
| 12:10~12:50 | 40’ | Comparison of GMP regulations for global blood establishments | TAE KYU KIM (BnP Care) |
|
GBC6
Human Tissues Forum
Forum Overview
| Date/Time | 9.14(Tue) 14:00 – 18:00 | Location | Grand Ballroom III (5F) |
|---|---|---|---|
| Overview |
Status of international and Korea safety management of human tissue in the COVID-19 era We will share several cases of aseptic process, including the collection and packaging raw materials of human tissue; the strategic method of setting a proper expiration date; and the purpose of use of human tissue. The discussion will also cover the international and Korea status of human tissue management in the COVID-19 era, including strategy to assess tissue donor suitability for safe human tissue management in times of novel infectious disease outbreak. |
||
Program
| Time | Program | Speakers | Link | |
|---|---|---|---|---|
| Status of international and Korea safety management of human tissue in the COVID-19 era | ||||
| 14:00~14:50 | 50’ | Kwang-In Lee (L&C BIO) |
||
| 14:50~15:40 | 50’ | TBD | Yang-Guk Chung (Seoul St. Mary Tissue Bank under Korea Public Tissue Bank) |
|
| 15:40~15:50 | 40’ | Break | ||
| 15:50~16:40 | 50’ | YOUNG JOO CHA (Institute Pasteur Korea) |
||
| 16:40~17:30 | 50’ | John Boyd (AATB) |
||
GBC7
Advanced Biopharmaceuticals & Combine Products Regulatory Science Forum
Forum Overview
| Date/Time | 9.14(Tue) 09:00 – 12:00 | Location | Grand Ballroom III (5F) |
|---|---|---|---|
| Overview |
Part 1: Current Development Trends and Regulatory Environments for Advanced Biopharmaceuticals We will look into the current development trends and the regulatory environments for Advanced Biopharmaceuticals. A further discussion will lead to how to develop reasonable policies in a rapidly changing environment. Part 2: Current Development Trends and Global Regulation Status on Combination Products Recently, the volume of the global combination product market sees the steady growth, and it has led the active development of combined products with advance biopharmaceuticals and medical devices. The forum shares the latest trend of development in Korea and overseas regulations on the combination product, especially focused on the advanced biopharmaceutical-combined product. |
||
Program
| Time | Program | Speakers | Files | |
|---|---|---|---|---|
| Part 1: Current Development Trends and Regulatory Environments for Advanced Biopharmaceuticals | ||||
| 09:00~09:30 | 30’ | YEON-SOO KIM (Chungnam National University) |
||
| 09:30~10:00 | 30’ | USP Standard Development for Advanced Therapies | Ben Clarke (USP) |
|
| Part 2: Current Development Trends and Global Regulation Status on Combination Products | ||||
| 10:00~10:30 | 30’ | Jinah Jang (POSTECH) |
||
| 10:30~11:00 | 30’ | John Weiner (FDA) |
||
GBC8
GMP Forum
Forum Overview
| Date/Time | 9.14(Tue) 14:00 – 18:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
Sharing information on non-face-to-face GMP inspection in the COVID-19 era As the field inspection found its limitations because of COVID-19, non-face-to-face GMP inspection has been prevalent in biopharmaceuticals industries globally in recent years. GBC 2021’s GMP Forum will further discuss the latest information on this new trend of contactless inspection cases in Korean biopharmaceuticals manufacturers and the requirements for the inspection to thoroughly manage safety. |
||
Program
GBC9
Global Vaccine Hub Strategy Forum
Forum Overview
| Date/Time | 9.15(Wed) 09:00 - 12:00 | Location | Grand Ballroom III (5F) |
|---|---|---|---|
| Overview |
Vaccine Hub Strategy of a Joint Response to the Global Pandemic in the Post-COVID-19 Era Based on the recognition of the importance of vaccine supply increasing for a joint response to the global pandemic in the post-COVID-19 era, we will introduce mid and long-term strategies and public and private sector cooperation in developing a hub for vaccine production and supply, and the possibility of establishing global production base utilizing Infrastructure of local vaccine production base. Further discussion will take place on future directions in preparation for the post-COVID-19 era, including government-wide policy support necessary for Korea to become a global vaccine hub |
||
Program
| 시간 | 프로그램 | 연사 | 자료 | |
|---|---|---|---|---|
| 09:30-09:50 | 20’ | You Joo Hun (Global Vaccine Hub Office of the Ministry of Health and Welfare) |
||
| 09:50-10:20 | 30’ | TBD | Baik-Lin Seong (COVID-19 Vaccine Initiative of Korean Government) |
|
| 10:20-10:40 | 20’ | Digital Transformation in Pharma | James Choi (Samsung Biologics) |
|
| 10:40-11:00 | 20’ | SK bioscience’s strategy of the global bio platform hub | HUN KIM (SK bioscience) |
|
| 11:00-11:20 | 20’ | Rethinking supply chain to emerge stronger from COVID-19: Cytiva’s commitment to Korean vaccine industry | Kihoon Kim (Cytiva Korea) |
|
| 11:20-11:35 | 15’ | Jeongsuk Lee (Korea Biomedicine Industry Association) |
||
| 11:35-11:40 | 5’ | Closing | ||
GBC10
Regulatory Workshop
Forum Overview
| Date/Time | 9.15(Wed) 09:00 – 12:00 | Location | Grand Ballroom II (5F) |
|---|---|---|---|
| Overview |
Current state and future directions of regulatory authorities We will be discussing regulatory authorities’ current attitudes toward technological changes and innovations in the biopharmaceutical industry. Also, the directions that authorities will be taking for its successful development and safe society will be discussed. Any industries participating can enroll for one-on-one meetings with global regulatory authorities upon preregistration. |
||
Program
BtoB1
Korea-ASEAN Pharmaceutical GMP Conference
Forum Overview
Registration for GMP Conference >
| Date/Time | 9.15(Wed) 14:00 – 17:00 | Location | Iris (2F) |
|---|---|---|---|
| Overview | Since 2015, the Ministry of Food and Drug Safety (MFDS) has been organizing the “Korea-ASEAN Pharmaceutical GMP Conference" to enhance mutual understanding and cooperation between Korea and ASEAN Member States in the field of pharmaceutical GMP through presentations, panel discussions and site visit. However, due to the prolonged COVID-19 pandemic, the 2021 Korea-ASEAN Pharmaceutical GMP Conference will be held virtually from September 14-15 2021. As the rapid development and supply of COVID-19 vaccines and treatments have been a global issue due to the COVID-19 pandemic, MFDS is organizing the ”2021 Korea-ASEAN Pharmaceutical Conference” in conjunction with Global Bio Conference (GBC) under the topic of “GMP Assessment of Biopharmaceuticals in Post COVID-19 Era.” This Conference is aimed to share knowledge and productive opinions related to various GMP assessment approaches in post COVID-19 era such as case studies of remote GMP inspections of biopharmaceuticals manufacturers and current status of GMP assessment based on mutual reliance among regulatory authorities. |
||
Program
| Time | Program | Speakers | Files | |
|---|---|---|---|---|
| Opening | ||||
| 14:00~14:05 | Opening remarks | Dr. Seogyoun Kang (MFDS) |
||
| 14:05~14:10 | Introduction and announcements | Facilitator (MFDS) |
||
| II. GMP Assessment Based on Mutual Reliance | ||||
| 14:10~14:30 | GMP inspection reliance from a PIC/S perspective | Mr. Boon Meow Hoe (PIC/S Immediate Past Chairman) |
||
| 14:30~14:50 | Status of GMP assessment based on mutual reliance - Korea | Ms. Mija Park (MFDS) |
||
| 14:50~15:10 | Introduction of ASEAN Mutual Recognition Arrangement on GMP inspection and Listed Inspection Service(LIS) | Belinna Abu Bakar (NPRA, Ministry of Health Malaysia) |
||
| 15:10~15:30 | Status of GMP assessment based on mutual reliance - ASEAN Member States : Singapore |
Prof. Chong Hock Sia (Singapore HSA) |
||
| 15:30~16:00 | Q&A | Facilitator (MFDS) |
||
| 16:00~16:20 | Break | |||
| III. Various GMP Assessment Approaches in Post COVID-19 Era (Panel discussion) | ||||
| 16:20~16:50 | ⚫Challenges in remote inspection and how to overcome them ⚫Information sharing between regulatory authorities based on mutual reliance : benefits, challenges, etc. |
Moderator (MFDS) Day 1: Speaker (Industry) Day 2: Speaker (Regulator) |
||
| Closing | ||||
| 16:50~16:55 | Closing remarks | Ms. Young-ah Kang (MFDS) |
||
| 16:55~17:00 | Closing | Facilitator (MFDS) |
||